Instructions:
Each year, 5-million tonnes of road salt is added to Canadian roads. This salt dissolves and makes its way into our lakes and rivers. Road salt is commonly made up of Sodium (Na) and Chloride (Cl). Since the chloride in road salt does not attach to soils, it can move down through the soil to the groundwater table. Howard and Haynes (1993) found that 55 percent of salt applied to roads in Toronto enters the shallow waters under the ground’s surface (soil water). Cusack (n.d.) estimated that approximately 45 percent of chlorides applied as road salt will be carried down deeper to the groundwater. Chloride entering groundwater systems is likely to stay for a long time since there is no easy way for it to be removed, and groundwater moves slowly.1
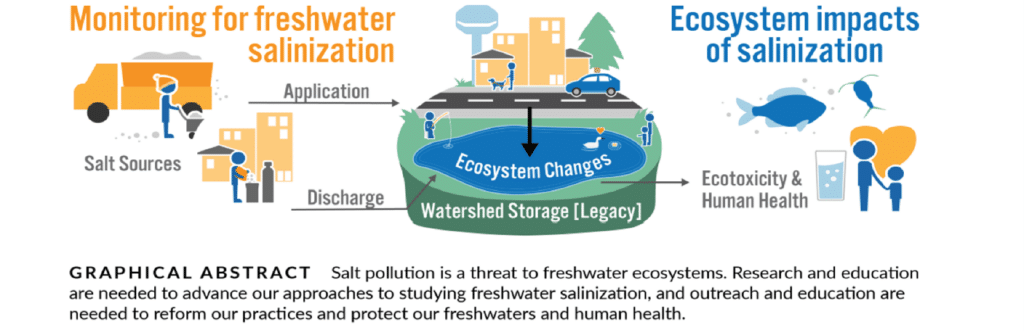
Graphic taken from the following study: Dugan, H & Arnott, S. (In Press). The ecosystem implications of salt as a pollutant of freshwaters.
Chloride is difficult to measure without specialized equipment, however, we can use other methods to indicate the presence of road salt. For instance, we can measure water hardness or the amount of ions in the water.
Students follow the instructions in the Testing for Water Hardness.pdf to complete the following experiment. Students will create hard and soft water and use their experimental method to test a sample of water from a nearby body of water. Through the experiment students will learn how to determine if water is hard or soft, therefore, showing if it contains a lot of minerals (like road salt).
1. In three jars/bottles with lids, students fill
- one with distilled water (soft water or water without minerals),
- one with distilled water and dissolved road salts (or table salt- 1 teaspoon per liter of water, to mimic the concentration of road salt in an urban pond), and one with water from a local stream/lake.
2. After the salts are completely dissolved, a small amount (several drops) of dish soap can be added to each, and the closed jar shaken vigorously.
3. Students make observations and based on results, estimate the hardness of the local water sample.
4. As an extension, in the wintertime, students can melt snow from different areas on the school yard (near the road vs. not) and test the hardness of water from these different ocations. After the soap test, the water can be left in an open jar for several days, or heated, to allow the water to completely evaporate. After all the water is gone, students can see the minerals left behind. Students should consider if this matches with the results they found with the soap test. As the students may have found, the amount of minerals in the water affects the ability of soap to make suds. Not only does this impact our cleaning tasks, these minerals in the water, also impact drinking water and the ecosystems they enter. This will be investigated more in the next activity.
5. Ask students to explore alternatives (ie: sand) that could be used in place of road salt and what is already different provinces
1 Information from “Environmental impacts of road salt and other de-icing chemicals”: https://rb.gy/u5maw



